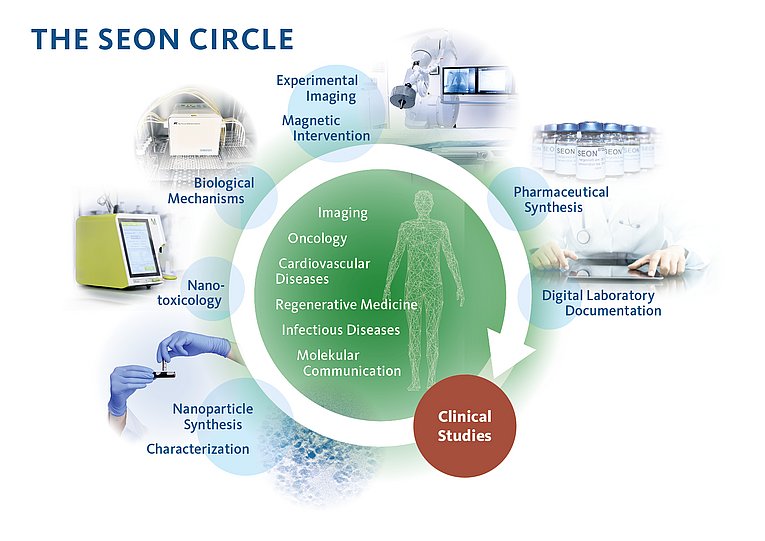
Translational research at SEON begins with a symbolizing circle. The nanomedical expertise that has distinguished SEON for over ten years comes to bear in researching the best possible diagnostic and therapeutic options and their use in everyday clinical practice. SEON uses superparamagnetic iron oxide manoparticles, which are very small and have a large surface area, as a means of transport for the targeted, magnetic field-controlled delivery of drugs. The SEON circle (see image) depicts the complete workflow along the nanoparticle systems research:
From nanoparticle fabrication and characterization and nanotoxicology to biological mechanisms in nanoparticle enrichment, experimental imaging and pharmaceutical manufacturing. The specific linkage of development and application goals is considered unique worldwide, and the resulting interactions are proving enormously fruitful. All SEON work areas are extremely closely interlinked, which benefits not only the workflow, but also the continuous improvement of nanoparticle systems in a wide variety of research fields.
Nanoparticle Fabrication
The main focus of nanoparticle synthesis is on the development of nanoparticles for different application areas, for example for therapy in the field of oncology, cardiovascular diseases and sepsis, as well as for the diagnostic field and for regenerative medicine. Depending on the area, different requirements are placed on the nanoparticles. Alkaline precipitation or thermal decomposition reactions serve as the basis for our syntheses, which can be used to produce magnetic iron oxide nanoparticles. We can colloidally stabilize these either during these processes or afterwards with various substances and load them with active ingredients. The binding of the active substances can be either adsorptive or defined via chemical bonds.
Physicochemical Characterization
In order to classify the corresponding effects of the formulations produced - intended therapeutic or unintended toxic - the particles must be analyzed in detail physicochemically with regard to their nature. An important parameter is the aggregate size of the particles, which is determined, for example, by dynamic light scattering (DLS) measurements. We can investigate the size and additionally the morphology of the individual particles by electron microscopic imaging. In this context, the electrostatic potential of particles in a suspension at the shear plane, the ζ-potential, is an important parameter for the stability of colloidal suspensions and is routinely recorded, as is the iron content by atomic emission spectroscopy. The active ingredient content of the formulations is determined using the liquid chromatography method with mass spectrometry coupling, and the particle surfacefl area is characterized by FTIR. Since SEON is integrated into the Cluster of Excellence "Engineering of Advanced Materials" (EAM), we use its equipment pool for more in-depth particle characterization studies.
Nanotoxicology
To achieve our goal of translating superparamagnetic iron oxide nanoparticles (SPIONs) from the laboratory to the clinic, extensive toxicological studies based on physicochemical characterization of the nanoparticles are an important prerequisite. Because nanoparticles interfere with numerous classical toxicological analytical systems, we have devised alternative methods for nanotoxicology. Thus, an extensive test battery has been developed in recent years, enabling us to answer numerous questions regarding the biocompatibility of nanoparticles. Complementary methods such as Durchflusscytometry or fluorescence microscopy, in which multiparameter staining with fluorescence-labeled markers is performed, as well as label-free real-time cell analyses using live-cell microscopy or impedance analysis are applied here. Overall, our toxicological testing tools enable an assessment of particle biocompatibility in realistic exposure scenarios. By means of feedback loops to the synthesis unit, we ensure ongoing optimization of particle formulations up to successful translation into the clinic.
Biological Mechanisms
The biological mechanisms of nanoparticle uptake and enrichment are intensively studied at SEON. However, different SPIONs often differ in their magnetic enrichment capacity. The behavior of circulating particles depends on the nanoparticle properties, magnetic field strength, and flow dynamics. Throughfluss cell culture slides are used to study the enrichment efficiency under physiologically similar conditions. To estimate the magnetic enhancement of SPIONs under arterial flow conditions, we created an improved ex vivo model of the human umbilical artery. Using this model, the magnetic enhancement of different SPION types under different external magnetic field gradients and flow conditions is investigated by atomic emission spectroscopy and histology. Similarly, we are exploring the effects of blood cells on the efficacy of magnetic capture.
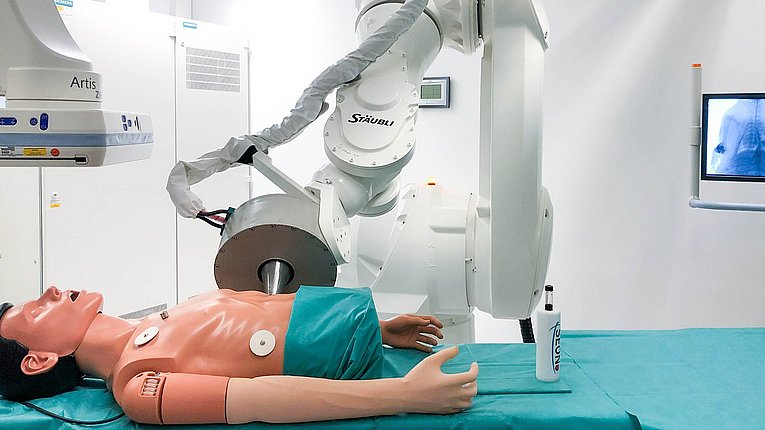
Experimental Imaging and Magnetic Intervention
SEON's stated goal is to bring iron oxide nanoparticles into clinical use. For this reason, we developed an animal model in rabbits very early on in the field of oncology with which we can perform magnetic drug targeting (MDT). Here, the tumor-supplying vascular bed is first visualized in 3D with the aid of contrast media. In the next step, we insert a catheter via the carotid artery, which is placed near the tumor using image control by the angiography system. Now we turn the table of the angiography unit towards the magnet, place it on the tumor and apply the nanoparticles with the magnetic field switched on. Superparamagnetic iron oxide nanoparticles (SPIONs) are capable of inducing signal cancellation in an MRI scanner. This ability can be used to verify whether and how many nanoparticles could be enriched in the diseased region and how they are distributed there. This so-called theranostic approach, i.e. the combination of therapy and diagnostics, is one of the major advantages of MDT with the aid of magnetic nanoparticles. In addition, our research group is trying to extend the theranostic approach to the imaging of nanoparticles using ultrasound in a cooperation with the Chair of Sensor Technology at the Friedrich-Alexander University Erlangen-Nuremberg. The first successes show that this - compared to MRI and MPI - relatively inexpensive method can not only image the nanoparticles, but that they can possibly even be quantified with the help of ultrasound.
Pharmaceutical Production
An essential part of the clinical translation process of drug candidates is the quality-controlled manufacturing according to valid manufacturing guidelines, called current good manufacturing practice (cGMP). In this context, the German Medicines Act (AMG) stipulates an implementing regulation. This regulates the quality criteria according to which approved medicinal products or investigational medicinal products must be manufactured if the active substance is still at the clinical trial stage. This guideline guarantees suitable manufacturing processes that deliver the required product quality. This means that all relevant synthesis steps are validated and qualified personnel, flawless equipment and infrastructure are used. Process controls and product testing are established and the entire manufacturing process, including analytics in the form of Standard Operating Procedures (SOPs), is defined. Such quality-controlled processes are very costly. It is therefore self-explanatory that cGMP only makes sense for promising developments from the basic research area. In addition, the technical feasibility of carrying out syntheses on a much larger production scale while retaining the product properties must be demonstrated. To achieve this, we perform granular parameterizations of all relevant process steps. These ultimately enable us to define a safety corridor for the manufacturing conditions under which we achieve the required product quality. Full implementation of cGMP-compliant synthesis cannot be achieved without trusting collaboration with strategic partners.