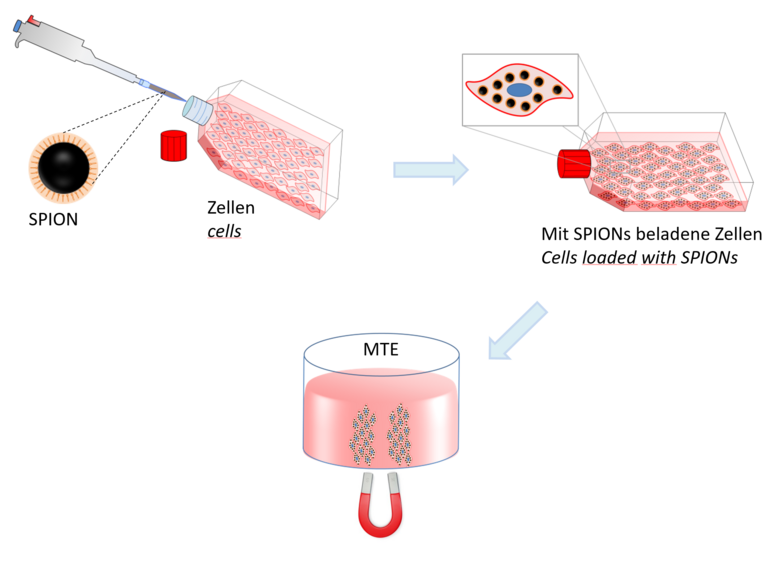
The voice is one of the most important instruments of interpersonal communication. The voice is essentially formed in the larynx, where a sound is produced by the vibration of the vocal folds. If the vocal fold is injured, for example during an operation, voice disorders or loss can occur, which is associated with an enormous reduction in the quality of life. To date, there is no permanent functional replacement in this field, as the complex structure of the vocal fold cannot be easily reconstructed. Regenerative medicine aims to repair or replace such tissues and organs that no longer function due to age or disease. One manifestation of regenerative medicine is the so-called Magnetic Tissue Engineering (MTE), with which we aim to generate functional vocal fold tissue in vitro. The principle of MTE is based on superparamagnetic iron oxide nanoparticles (SPION) being taken up by cells, which are then directed and influenced by a magnetic field in such a way that they form a three-dimensional structure (see picture). In this way, a functional multilayer tissue can be produced. This should be the basis for the translation of this method into humans, in order to reproduce an individually formable, personalized and functional vocal fold. This should restore the verbal communication ability of patients with vocal fold defects and thus significantly improve their quality of life.
Functional Tissue Engineering of the Human Vocal Fold with SPIONs - Magnetic Tissue Engineering (MTE).
Else Kröner-Fresenius Foundation, 2020-2022
Project goal
In the context of the project described here, the reconstruction of human vocal fold tissue will be achieved by means of nanotechnology, more precisely by Magentic Tissue Engineering (MTE). The aim of this project is the translation of this method into the human system, which will be achieved by creating a functional autologous vocal fold graft from human cells. This should restore the verbal communication ability of patients with vocal fold defect after surgery and thus significantly improve the quality of life.
Collaboration partner
- Dr. Christian Braun, LMU Munich
Field-driven particle-matrix interactions: Generation, cross-scale modeling and application of magnetic hybrid materials (SPP 1681)
SPP DFG-Funding, 2013-2020
Project goal
Five key questions are at the center of this priority program: First, it must be clarified how (1) the material behavior of a magnetically controllable hybrid material can be influenced via the particle-matrix interaction and how corresponding materials can be synthesized. Understanding the material behavior requires (2) a cross-scale consistent description that explains the magnetic controllability of the material properties on a microscopic basis. This material modeling is also required to generate material laws for application based on a detailed understanding of materials. Closely related to the descriptive concepts is (3) the experimental investigation of material behavior in the magnetic field, and thus what changes in material properties can be produced by varying their internal structure in the magnetic field. Building on the understanding of hybrid magnetic materials, it is then possible to address both (4) what opportunities they offer in novel actuator and sensing applications, and (5) how to improve the effectiveness of the biomedical use of magnetic nanoparticles by controlling the interaction between functionalized particles and tissue.
Collaboration partner
- Prof. Dr. Stefan Odenbach, TU-Dresden
- Prof. Dr. Lutz Trahms, PTB Berlin
- Dr. Frank Wiekhorst, PTB Berlin
- Prof. Dr. Stefan Mayr, IOM Leipzig
- Prof. Dr. Annette Schmidt, Universität zu Köln
- Prof. Dr. Hartmut Löwen, Universität Düsseldorf
- Dr. Andreas Menzel, Universität Düsseldorf
- Prof. Dr. Silvio Dutz, TU-Ilmenau
- Dr. Joachim Clement, Universitätsklinikum Jena
- Prof. Dr. Meinhard Schilling, TU Braunschweig
- Dr. Thilo Viereck, TU Braunschweig
Cell colonization of electrospun biocomposite scaffolds for vascular graft tissue engineering
Staedtler Stiftung, 2019
Project goal
The aim of this study is to investigate the feasibility of fabricating a small diameter three-dimensional vascular prosthesis by using (a) electrospinning of biodegradable polymers and (b) a magnetic cell transfer technique. This method is tested on scaffolds composed of electrospun PCL with or without silk fibroin. The three-dimensional TE constructs will be thoroughly investigated with respect to their cell-material interactions after cell transfer of primary EC, SMC and/or fibroblasts. In addition to mechanical properties, adhesion, growth, proliferation, and metabolic activity of the transferred cells will be studied in detail to gain insights into the properties of TE-based better patency prostheses and their suitability as vascular grafts in patients with cardiovascular diseases.
Collaboration partner
- Prof. Dr. Aldo R. Boccaccini, FAU Erlangen-Nuremberg
- Dr. Liliana Liverani, FAU Erlangen-Nuremberg
- Dr. Barbara Dietel, UK Erlangen
Development of a vocal fold transplant for voice rehabilitation after surgical tumor therapy
Deutsche Krebshilfe e.V., 2014-2017
Project goal
In this project the isolation and cultivation of epithelial cells and fibroblasts of the vocal fold of the rabbit will be established. With the help of SPION, a 3D cell culture will be created, which, when examined in vitro, will allow much more meaningful conclusions to be drawn about the actual situation in vivo than the 2D cell culture. The first goal of this project is to produce a three-dimensional vocal fold graft in vitro and implant it into a rabbit larynx. By considering the anatomically and physiologically real laryngeal configurations in the rabbit model, the vibration characteristics after implantation of the vocal fold graft will be compared to untreated rabbit larynxes. The second goal is the biomechanical testing and analysis of the vocal fold graft on the excised rabbit larynx in the laboratory of the Department of Phoniatrics and Pediatric Audiology. For this purpose, both vocal fold movement and the acoustic voice signal of the rabbit larynx without treatment and after implantation of a vocal fold graft will be investigated. The knowledge gained will be used to create conditions in the larynx that are conducive to normal vocalization or make it possible again through the use of a vocal fold transplant.
Collaboration partner
- Prof. Dr. Michael Döllinger, UK Erlangen
- Dr. Antoniu-Oreste Gostian, UK Erlangen
- Dr. Matthias Balk, UK Erlangen
- Dr. Konstantinos Mantsopoulos, UK Erlangen
- Prof. Dr. Werner Lang, UK Erlangen
- Dr. Susanne Regus, UK Erlangen
- Prof. Dr. Harald Mangge, Universität Graz
- Prof. Dr. Matthias Graw, LMU Munich
- Prof. Dr. Oliver Friedrich, FAU Erlangen-Nuremberg
- Prof. Dr. Christian Pilarsky, UK Erlangen
- Prof. Dr. Markus Gugatschka, Universität Graz